 Laboratory for Zero-Carbon Energy,
Laboratory for Zero-Carbon Energy,
Institute of Innovative Research, Tokyo Institute of Technology
Associate Prof. Hiroki TAKASU
Due to concerns about global warming and increasing abnormal weathers worldwide, the movement towards achieving carbon neutrality has accelerated in recent years. As of October 2022, over 150 countries and regions have already announced their commitments to achieve carbon neutrality within a specified timeframe[1]. As the value of carbon neutrality becomes more widely shared, some new technologies are emerging as necessary. For example, the expansion of renewable energy deployment has made it possible to utilize electricity affordably, sometimes even as surplus energy, without concerns about instability or depletion. This has led to a shift from the traditional view that even the slightest inefficiency in the use of expensive electric energy is unacceptable, to a more realistic view that the introduction of technologies that enable energy reuse, even in partial forms, will lead to the effective use of excess energy that would otherwise be wasted.
As an example, in Japan, the Fukushima Hydrogen Energy Research Field (FH2R), the country's largest water electrolysis facility, was established in 2020[2]. It is well known, according to the law of energy conservation, that the energy available from hydrogen production via water electrolysis decreases compared to the input energy (approximately a 20% reduction due to conversion losses alone). Therefore, water electrolysis is fundamentally an unnecessary process that wastes energy. However, with the increasing disposal of surplus energy due to the expansion of renewable energy, it has become an effective option to store and reuse this wasted energy across time and space.
Now, while there is still room for research and development towards practical applications, our laboratory is focusing on the electrolysis of carbon dioxide (CO2) as a promising technology candidate for the future. In carbon neutrality, the goal is to achieve a virtual zero emission of greenhouse gases such as CO2. Negative emission technologies are currently being considered as a solution for industries and transportation sectors where it is not practically feasible to eliminate carbon use entirely. On the other hand, carbon is utilized not only as an energy source but also in various materials used in everyday life, and its use is expected to continue in the future. Therefore, it is necessary to consider both the emission and supply of carbon. CO2 electrolysis, capable of equalizing the amount of carbon supplied and emitted, is exactly the technology needed. In most cases, carbon is ultimately emitted as CO2. To reuse carbon, it is necessary to chemically convert this stable CO2 into a substance with higher energy status by applying some form of energy input. For example, by converting CO2 into high-value-added substances such as carbon monoxide or formic acid, it can be reused as a clean raw material for chemical products and fossil fuels. Although various CO2 conversion technologies are conceivable, CO2 electrolysis utilizing high-quality electrical energy, which can be easily controlled in terms of voltage and current, can be considered a promising technology that can meet the demands of large-scale CO2 conversion required by industrial and social sectors.
Our laboratory is currently working on the development of solid oxide electrolytic cells (SOECs) with a view toward larger scale production (Figure 1). Specifically, we are developing metal-supported SOECs (Figure 2) with a unique structure that utilizes a metal wire mesh as a support and introduces a reaction layer through thermal spraying[3]. Using metal as a support enables us to address challenges such as large-area (high-capacity) cell production and cost reduction in existing cells, while also expecting improvements in startup speed during operation. In fact, we have manufactured coin-type cells with a diameter of 2 cm and reported the principle demonstration of CO2 high-temperature electrolysis[4]. Currently, we are working on improving cell performance, enhancing durability, and scaling up cell capacity. In addition, at The GXI Ookayama Laboratory, we have introduced a large-scale cell (100 cm2 class) evaluation system and plan to introduce research progress and our participation in the GXI activities to participating companies through public events.
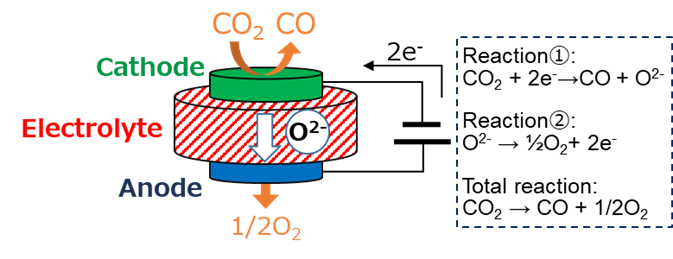
Figure 1 Basic reaction overview of CO2 electrolysis using SOEC
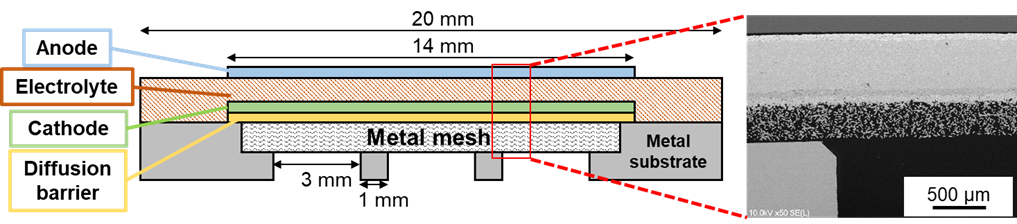 Figure 2. A conceptual diagram and microscope SEM image illustrating the cross-sectional structure of the metal-supported solid oxide electrolysis cell (SOEC) under development in the laboratory
Figure 2. A conceptual diagram and microscope SEM image illustrating the cross-sectional structure of the metal-supported solid oxide electrolysis cell (SOEC) under development in the laboratory
Reference
[1] The Ministry of Economy, Trade and Industry of Japan, The Annual Report on Energy, June 6, 2023
https://www.meti.go.jp/english/press/2023/0606_003.html
[2] The New Energy and Industrial Technology Development Organization (NEDO), The world's largest-class hydrogen production, Fukushima Hydrogen Energy Research Field (FH2R) now
The world's largest-class hydrogen production, Fukushima Hydrogen Energy Research Field (FH2R) now is completed at Namie town in Fukushima. March 7, 2020 (accessed on 2024/02/20)
https://www.nedo.go.jp/english/news/AA5en_100422.html
[3] Y. Numata, K. Nakajima, H. Takasu, Y. Kato, Carbon Dioxide Reduction on a Metal-Supported Solid Oxide Electrolysis Cell, ISIJ International, 59, 4, 628-633, 2019
[4] H. Takasu, Y. Maruyama, Y. Kato, Development of Metal Supported SOEC for Carbon Recycling Iron Making System, ISIJ International, 60, 12, 2870-2875,. 2020


