 Tokyo Institute of Technology, Prof. Yoichi MURAKAMI
Tokyo Institute of Technology, Prof. Yoichi MURAKAMI
As predicted by the Intergovernmental Panel on Climate Change (IPCC) [1], increasing greenhouse gases (GHGs) are causing climate change and posing various risks worldwide. concentration is increasing at an accelerating rate (417 ppm in 2022, where ppm is the millionth fraction) [3]. According to the above report, net CO2 emissions from 1850 to 2019 were 2400 ± 240 gigatons, 58% of which were emitted in the roughly 140 years from 1850 to 1989, while 42% were emitted in just about 30 years from 1990 to 2019 [1]. This means that humans have emitted an average of about 35 gigatons of CO2 per year (about 35 billion tons) over the last 30 years.
The term carbon neutrality (CN) means balancing the emissions and removals of carbon-containing molecules that are GHGs to achieve zero net emissions [4]. According to the International Energy Agency's roadmap [5], to achieve CN in 2050, 4 gigatons of CO2 will need to be captured in 2035 and 7.6 gigatons in 2050. And CO2 capture requires energy input. However, there is currently no technology that can separate and capture this enormous amount of CO2 with a realistic energy input. The technology for CO2 capture by absorption of CO2 into an aqueous solution of amine molecules, which is now in practical use, is superior in that it can be applied to emission sources of various sizes (scalability), but because it uses an aqueous solution, it requires a large input of heat energy for the regeneration process that removes CO2 from the amine molecules, and it also requires a large input of energy for the recovery of CO2 from the amine solution. However, the use of an aqueous solution requires the input of a large amount of heat energy for the regeneration process to strip CO2 from the amine molecules, the aqueous amine solution is highly corrosive, and the amine molecules are easily volatilized and degraded[6]. The excessively high heat of reaction between the amine used and CO2 is also a cause of the need for a large amount of energy input for regeneration [7].
Two points are important in solving this problem. The first is to discuss CO2 separation and recovery based on a proper understanding of thermodynamics, which is a scientific principle, and to consider the technology. Recently, there are often claims of technologies that do not consider the most important process, regeneration, or that effectively remove it from the scope of discussion. As the author explains in a mock lecture for the general public [8], understanding this point is the starting point for sound technology consideration. The second point is the effort to reduce the energy input required for regeneration by using solid CO2 absorbers. In recent years, the development of such solid-based CO2 adsorbents has been unfolding in cutting-edge research areas [6,7,9].
Our research group is developing a new generation of porous materials called covalent organic frameworks (COFs) as high-performance solid adsorbents. We have strengths in the synthesis of COFs, for example, we have recently discovered how to control the topology of the framework [10], and COFs are designable crystalline porous materials that not only have internal diffusion pathways for CO2 molecules, but are also believed to be capable of chemisorbing CO2 on the framework (Figure 1). Through the creation of such innovative materials, we are pursuing solutions to the problems common to all humankind mentioned at the beginning of this paper. If we can create separation and recovery technologies using such materials, it will lead to the creation of industries on a global scale. We are currently seeking corporate collaborators to help us realize this goal.
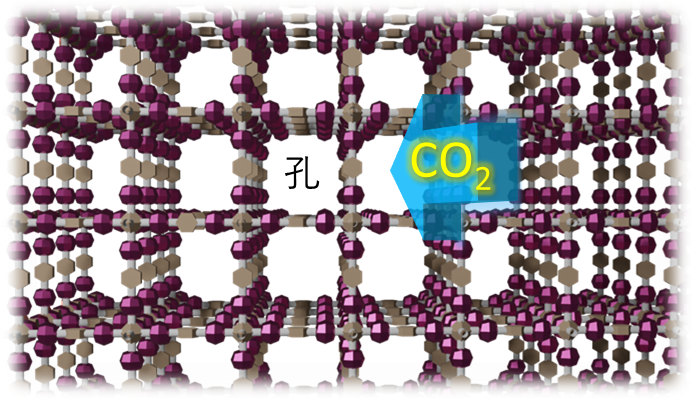
Figure 1 Conceptual schematic of CO2 capture using a covalent organic framework (COF); COF is a crystalline porous material with nanopores, and the framework has a CO2 adsorption function.
[1] Intergovernmental Panel on Climate Change (IPCC), "Climate Change 2023 Synthesis Report¾Summary for Policymakers" URL: https://www.ipcc.ch/report/ar6/syr/
[2] United States Environmental Protection Agency, "Overview of Greenhouse Gases" URL : https://www.epa.gov/ ghgemissions/overview-greenhouse-gases
[3] National Oceanic and Atmospheric Administration (NOAA), "Climate Change: Atmospheric Carbon Dioxide" URL: https:// www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide
[4] European Parliament, "What is carbon neutrality and how can it be achieved by 2050?" URL: https://www.europarl.europa. eu/topics/en/article/20190926STO62270/what-is-carbon-neutrality-and-how-can-it-be-achieved-by-2050
[5] International Energy Agency, "Net Zero by 2050¾A Roadmap for the Global Energy Sector" URL : https://www.iea.org/ reports/net-zero-by-2050
[6] Nature Materials, vol. 20, pp. 1060-1072, 2021. URL: https://doi.org/10.1038/s41563-021-01054-8
[7] Advanced Functional Materials, vol. 33, pp. 2213915-1-2213915-9, 2023. URL: https://doi.org/10.1002/adfm.202213915
[8] Yoichi Murakami, A Lecture in Tokyo Tech Open Campus 2023 (in Japanese), "The Problem of Increasing CO2 Concentration and the Thermodynamics That Determines Its Capture Efficiency" URL: https://youtu.be/9u3-jzXUtVg
[9] Journal of the American Chemical Society, vol. 145, pp. 17151-17163, 2023. URL: https://doi.org/10.1021/jacs.3c03870
[10] Journal of the American Chemical Society, vol. 146, pp. 1832-1838, 2024. URL: https://doi.org/10.1021/jacs.3c13863


