Introducing sugars into a covalent organic framework to create a solid heat storage material for waste heat near 150°C that solves the conventional supercooling problem.
Introducing sugars into a covalent organic framework to create a solid heat storage material for waste heat near 150°C that solves the conventional supercooling problem.
Next-generation solid heat storage material consisting of only low environmental impact and abundant light elements
Main point
- There is a large amount of waste heat in the world at 100 to 200°C, but this energy is not being used effectively.
- Sugar alcohols (melting point 100-200°C) are a low-cost, low environmental impact phase-change thermal storage material.
- The problem with sugar alcohols was that they involve strong supercooling and become liquid (lose their shape) at the melting point.
- A new type of solid heat storage material that retains its shape even above its melting point has been developed by introducing mannitol, a type of sugar alcohol, into the "covalent organic framework" of a nanoporous material composed of light elements.
- We have solved the long-standing problem of supercooling and created a heat storage material that maintains the quality of the stored thermal energy.
Summary
Tokyo Institute of Technology, Institute of Innovative Research Yoichi Murakami of the Zero Carbon Energy Laboratory Professoret al. have developed a low-cost, safePhase-change thermal storage material [term 1].be (formal, literary)Sugar alcohol (a type of sugar) [term 2].which is a crystalline nanoporous material.Covalent organic skeleton [term 3].The company has created a solid heat storage material for use at temperatures around 150°C, which solves the various problems of sugar alcohols that had not yet been solved.Although sugar alcohols are inexpensive, safe, plentiful, and have low environmental impact, their freezing point (= temperature at which heat is extracted) is about 50-100°C lower than their melting point (= temperature at which heat is stored), and the temperature at which heat can be extracted is difficult to predict because the temperature at which solidification occurs is random (= supercooling problem). Such "strong supercooling" is based on the principle that "the higher the temperature, the higher the quality of thermal energy[Note 1]This is an unsolved problem that could undermine the significance of heat storage because the quality of the stored heat energy is significantly degraded from the perspective of the material. Furthermore, a common problem with many phase-change heat storage materials is that they lose their shape at the melting point (i.e., they lose their particle shape and become a large mass after solidification), which is a problem not only from a handling perspective but also from the perspective of increasing the heat conduction distance from the interior to the surface of the material, thereby slowing down heat exchange.
To solve this longstanding problem, Prof. Murakami et al. pursued the idea of impregnating a covalent organic skeleton crystal powder with mannitol, a type of sugar alcohol, to create a new-generation solid heat storage material composed only of light elements (carbon, hydrogen, nitrogen, and oxygen) that has no resource constraints and low environmental impact. As a result, the problem of supercooling of sugar alcohols, which had been an unsolved problem, was solved, and an epoch-making solid heat storage material was created that does not degrade the quality of stored heat energy. This achievement is expected to contribute to society's CO2emission reduction. Since a myriad of types of covalent organic skeletons are possible, this result represents the creation of a basic concept that has potential for development. These results were published on August 14 in the Royal Society of Chemistry (U.K.) journalMaterials HorizonsPublished online in.
Background
Most primary energy is used as heat. Thermal energy decreases in temperature from its source downstream (environment), but the "quantity of thermal energy" is not lost due to the law of conservation of energy. However, the usefulness of thermal energy, or "quality of thermal energy," is impaired as the temperature decreases, and when the temperature drops to about the same level as the environment, the usefulness of the thermal energy is lost. In other words, thermal energy at the same temperature as the environment is worthless. For example, the environment has enormous thermal energy, but it cannot be converted into useful work (e.g., electricity).[Note 1]The following is a list of the most common problems with the
Therefore, it is important to reuse a certain thermal energy for another use in the process of lowering its temperature, rather than immediately dumping it into the environment when finished using it at a high temperature. The technology to adjust the space at that time is "heat storage. There is an enormous amount of waste heat in the world, especially in the 100 to 200°C range, and it is important to use it for other purposes.[Ref. 1].Through Its Effective Use, Society's CO2emission reductions is becoming increasingly necessary.
The use of phase-change thermal storage materials is effective for high-density heat storage. Important aspects of phase-change thermal storage materials are (1) low cost, (2) no resource constraints, (3) safe, harmless, and easy disposal, (4) high thermal energy density, (5) high durability (slow degradation), and (6) solidification (heat release) without significant supercooling. Recently, sugar alcohols (a type of sugar) have begun to be actively studied as a phase-change heat storage material that satisfies conditions (1) to (4). This is a safe and inexpensive material that is naturally derived and is added to foods and medicines. Recent studies have shown that embedding sugar alcohols in porous silica with nanoscale pores can significantly improve the problem (5).[Ref. 2]. However, the problem (6), which reduces the quality of stored thermal energy and makes the temperature at which heat can be extracted difficult to predict, remains an unsolved problem.
It is often desirable for phase change heat storage materials to remain solid, especially in particle form, even above the melting point. This is because when the melting point is reached, the initially particulate material melts and loses its shape, and when it solidifies, it becomes a large mass. In other words, when a fluid (gas or liquid) is used as a heat exchange medium in an application, if the phase change heat storage material retains its particle shape throughout the period of use, the heat transfer resistance inside the particles will be small (because the distance from the center of the particles to the surface is short) and the contact area with the fluid will remain high because the powder has a large surface area. Also when systematizing,Fixed floor [term 4].It is also possible to use a device that allows a heat exchange fluid to pass through the voids between the particles, such as Thus, a phase-change heat storage material in solid form that maintains its particle shape even above the melting point has been desired.
Research Outcomes
Murakami Prof.et al. conceived the idea of creating a solid heat storage material that meets the above requirements by impregnating a crystalline nanoporous Covalent Organic Framework (COF) with a sugar alcohol. To demonstrate this, as COF, the research group had previously developed a method to synthesize large single crystals (>0.1 mm) of COF-300[Ref. 3]were selected. After trying several sugar alcohols, mannitol (melting point 167°C, c6H14O6) was impregnated into COF to create the targeted solid heat storage material (Figure 1a). Specifically, when mannitol was brought into contact with COF-300 crystals above its melting point, COF was found to spontaneously absorb the mannitol melt while deforming its crystal shape (expansion in the width and contraction in the longitudinal direction) (Figure 1b). This has created a composite heat storage material consisting of a sugar alcohol and COF single crystal that retains its particle shape above the melting point. X-ray structural analysis of the composite crystals revealed that they are impregnated with mannitol up to a COF to mannitol weight ratio of 1:0.77 (see Publicationsinformation below for details).
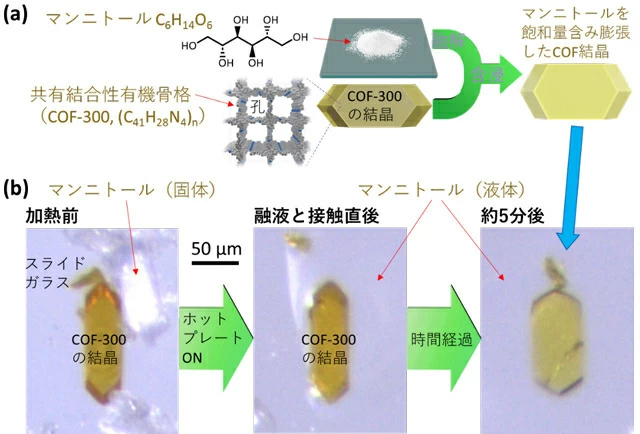
Figure 1. material creation of the invention. (a) Schematic of the conception, (b) Photographs taken under a microscope during the fabrication experiment.
The phase change properties of the materials were then evaluated using a differential scanning calorimetry minanalysis (DSC) device. First, the DSC results for mannitol only are shown in Figure 2a. During the temperature increase process, an endothermic (downward) peak representing melting appeared at 167°C, as is known. In the descending temperature process, an exothermic peak representing solidification appeared in the region of 85-110°C at random onset temperatures, and the strong undercooling described above was observed.
Next, to evaluate this composite material, a measured sample was prepared by placing a mixture of mannitol powder and COF crystals in a weight ratio of 1:1 in a sealed container and heating it above the melting point in inert gas. The result was a mixed material in which 34% of the mannitol was impregnated in the COF crystals and the remaining 66% remained outside the COF crystals. The DSC results (Figure 2b) showed that in addition to the endothermic heat derived from the mannitol outside the COF, a new endothermic heat in the region of 145-155°C and an exothermic heat in the region of 135-145°C appeared with repeatability. Furthermore, when the cycle of temperature increase was terminated at 160°C and temperature decrease was initiated (Fig. 2c), the mannitol-derived endotherm outside the COF disappeared, leaving only the newly emerged endotherm. These results indicate that these endotherms are derived from the mannitol introduced into the COF.
In conclusion, we have created a new sugar alcohol-based heat storage material that can repeatedly and reproducibly transfer heat in and out over a narrow temperature range without strong supercooling (Fig. 2a), which has been a problem in the past. This created a solid heat storage material with minimal deterioration in the quality of the stored heat energy. The temperature of endothermic heat can be tuned by changing the type of sugar alcohol and COF.
It is important to clarify the heat transfer characteristics of this material in order to develop its application as a heat storage material. The heat transfer properties of this material were measured in the laboratory of Junko Morikawa Prof.in the School of Materials Science and Engineering at the same university. As a result, the thermal diffusivity in the width direction (right side of Figure 1b) of the mannitol-impregnated COF-300 single crystal was found to be 2.3 ± 0.6 × 10-7m2/s (room temperature), which is higher than that of typical resin materials commonly used in industry (1 to 1.7 × 10-7m2/s, room temperature [Ref. 4] ) with a thermal diffusivity higher than that of the typical resin materials used in industry (1 to 1.7 × 10-7 m2/s, room temperature [Ref.
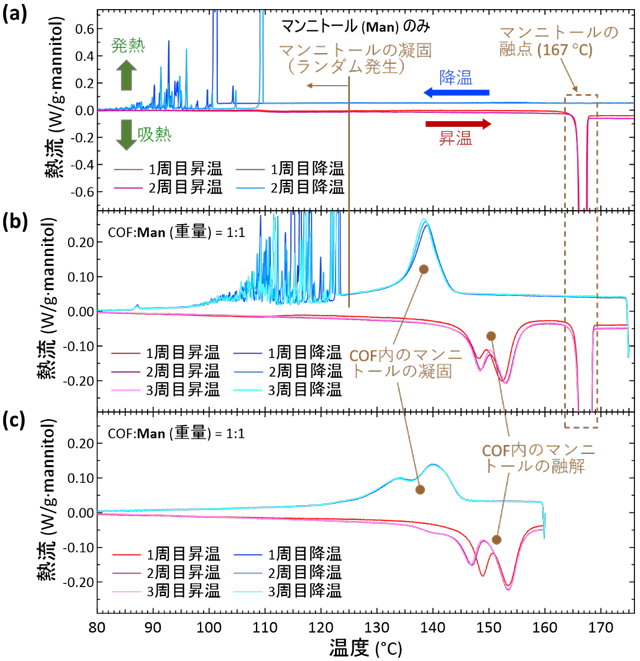
| 図2. | Differential scanning calorimetry minanalysis (DSC) results. Downward convexity is endothermic, upward convexity is exothermic. Temperature change is 2°C/ min. (a) DSC results for mannitol (Man) only. Melting point occurs at 167°C, whereas solidification occurs randomly in the region of 80-110°C, producing strong undercooling. (b) DSC results of a collective sample (several milligrams) of COF-300 single crystals impregnated with mannitol (Man). In this sample, part of the Man (about 1/3) is impregnated in the COF crystal, but the rest is present without entering the COF. (c) Cycle in which the DSC temperature increase was completed at 160°C and the temperature decrease was started from there; the phase change signal of Man outside the COF disappeared, and only the phase change of Man introduced into the COF could be selectively expressed. This created a solid heat storage material that repeatedly melts and solidifies reproducibly over a narrow temperature range. |
Social Impact
This invention solves the previously unsolved problem of supercooling of sugar alcohols, which have ideal characteristics as phase-change heat storage materials, such as low cost, safety, low environmental impact, and an industrially important melting point in the 100-200°C range. Furthermore, it has created an innovative heat storage material that maintains its particle form (does not lose shape) even above its melting point. This has promoted more advanced multistage use of primary energy, which is mostly consumed in the form of heat, and has helped society to reduce CO2emissions.
Future Development
Although this achievement opens up a new genre of solid heat storage materials and solves the longstanding problem of supercooling of sugar alcohols, in the combination of COF-300 and mannitol used for this proof-of-concept, the heat storage capacity per unit weight of mannitol is reduced to about 1/3 that of normal mannitol due to the nanoconfinement effect of the COF pores. The amount of heat stored per unit weight of mannitol was reduced to about 1/3 of that of normal mannitol. This issue can be improved by appropriately designing the interaction between mannitol and COF in the future. We will pursue the improvement of the performance of this technology through various joint research projects for its implementation in society.
Addition
The material creation and basic characterization were supported by JST PRESTO (JPMJPR18I9, FY 2018-2022, Murakami) and Grant-in-Aid for Scientific Research (22K18286, FY 2022-2026, Murakami), and heat transfer characterization by JST CREST (JPMJCR19I3, FY 2019-2024, Morikawa) and Grant-in-Aid for Scientific Research (22H02137, FY2022-2024, Morikawa).
Glossary
[term 1]. Phase change heat storage material : A material absorbs the latent heat of fusion when it melts from the solid phase to the liquid phase and releases the latent heat of solidification (usually equal to the latent heat of fusion) when it solidifies from the liquid phase to the solid phase. A material that stores thermal energy from a waste heat source at the melting point and releases the stored heat at the solidification point by utilizing such phase-change latent heat is called a phase-change heat storage material.
[term 2]. Sugar alcohol : A naturally occurring carbohydrate and a type of sugar. The name includes the word "alcohol" because each carbon has a hydroxyl group (-OH group) attached to it. There are various types, but the melting point is usually in the range of 100 to 200°C, which is an issue for recycling due to the large amount of waste heat. Xylitol and erythritol, which are used as sweeteners, are also sugar alcohols. In this study, mannitol (C6H14O6, melting point 167°C, Figure 1(a)) was used.
[term 3]. Covalent Organic Framework (COF): A crystalline organic solid with nanoscale pores and periodic framework formed by covalently linking building block minmolecules, which are the basic units, through polycondensation.[Ref. 5]The porous organic genre, which has been rapidly gaining attention in recent years, has been Virtually unlimited variety is possible through the selection of building blocks.
[term 4]. Fixed bed : A form of equipment in which a rigid container is filled with particles and the desired process is carried out by allowing fluid to flow through the container without changing the position of the particles. For example, the Japan Society of Mechanical Engineers Relevant entries in the Mechanical Engineering DictionarySee also.
Annotation
[Note 1] The usefulness of thermal energy depends not only on its quantity, but also on its quality. The same amount of thermal energy (e.g., 100 J) will be of higher quality if it is at a higher temperature, because a greater proportion of that amount can be converted into work (e.g., electricity). This rate is determined by the second law of thermodynamics. For example, 100 J of thermal energy in the environment (room temperature) has no ability to produce work and therefore has zero utility. From this perspective, if a certain amount of thermal energy is stored at a high temperature, but it is withdrawn at a lower temperature (> room temperature), then the quality of the stored thermal energy has deteriorated. Strong undercooling in phase-change thermal storage materials causes such a deterioration in the quality of thermal energy.
References
[1] Energy Technology, 2020, 8, 2000413
[2] Materials Chemistry and Physics, 2014, 146, 253
[3] Chemical Communications, 2021, 57, 6656
[4] Polymer Testing, 2005, 24, 628
[5] Science, 2005, 310, 1166
Publications Information
- Journal :.
- Materials Horizons (IF=13.3)
- Publications Title :
- Composite formation of covalent organic framework crystals and sugar alcohols for exploring a new class of heat-storage materials
- Author :.
- Y. Murakami, S. Mitsui, S. Nakagawa, X. Wang, H. Fujisawa, M. Ryu, and J. Morikawa

